Capsule is one of the ancient dosage forms of drugs, which originated in ancient Egypt [1]. De Pauli, a pharmacist in Vienna, mentioned in his travel diary in 1730 that Oval capsules were used to cover up the bad smell of drugs to reduce the pain of patients [2]. More than 100 years later, pharmacists Joseph Gerard Auguste dublanc and Francois Achille Barnabe motors obtained the patent of the world’s first gelatin capsule in 1843 and continuously improved it to adapt to industrial production [3,4]; Since then, many patents on hollow capsules have been born. In 1931, Arthur Colton of Parke Davis company successfully designed and manufactured the automatic production equipment of hollow capsule and produced the world’s first machine-made hollow capsule. Interestingly, up to now, the hollow capsule production line has only been continuously improved on the basis of Arthur’s design to improve product quality and production efficiency.
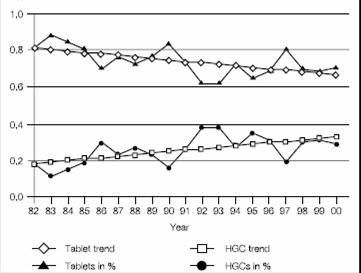
At present, capsule has made great and rapid development in the field of health care and pharmacy, and has become one of the main dosage forms of oral solid preparations. From 1982 to 2000, among the new drugs approved worldwide, hard capsule dosage forms showed an upward trend.
Figure 1 Since 1982, new molecular drugs have been compared between capsules and tablets
With the development of pharmaceutical manufacturing and R & D industry, the advantages of capsules have been more recognized, mainly in the following aspects:
1. Patient preferences
Compared with other dosage forms, hard capsules can effectively cover up the bad smell of drugs and are easy to swallow. Various colors and printing designs make drugs more recognizable, so as to effectively improve the compliance of drugs. In 1983, a survey conducted by European and American authorities showed that among the 1000 patients selected, 54% preferred hard capsules, 29% chose sugar coated pellets, only 13% chose tablets, and another 4% did not make a clear choice.
2. High R&D Efficiency
The 2003 tufts report pointed out that the cost of drug research and development increased by 55% from 1995 to 2000, and the average global cost of drug research and development has reached 897 million US dollars. As we all know, the earlier drugs are listed, the longer the market monopoly period of patented drugs will be, and the new drug profits of pharmaceutical enterprises will increase significantly. The average number of excipients used in capsules was 4, which was significantly reduced compared with 8-9 in tablets; The testing items of capsules are also less, and the cost of method establishment, verification and analysis is almost half that of tablets. Therefore, compared with tablets, the development time of capsules is at least half a year shorter than that of tablets.
Generally, 22% of new compound entities in drug research and development can enter phase I clinical trials, of which less than 1 / 4 can pass phase III clinical trials. The screening of new compounds can effectively reduce the cost of new drug research and development institutions as soon as possible. Therefore, the world hollow capsule manufacturing industry has developed preclinical capsules (pccaps) suitable for rodent trials ®); Precision micro filling equipment (xcelodose) suitable for the production of clinical capsule samples ®), And clinical double-blind capsules (dbcaps) suitable for large-scale clinical trials ®) And a full range of products to support Reducing R & D costs and improving R & D efficiency.
In addition, there are more than 9 types of capsules in different sizes, which provides multiple choices for the design of drug dose. The development of preparation technology and related equipment also makes the capsule suitable for more compounds with special properties, such as compounds insoluble in water. The analysis shows that 50% of the new compound entities obtained through high-throughput screening and combinatorial chemistry are insoluble in water (20%) μ G / ml), both liquid filled capsules and soft capsules can meet the needs of this compound preparation.
3. Low production cost
Compared with tablets, the GMP production workshop of hard capsules has the advantages of less process equipment, high space utilization, more reasonable layout, less inspection times in the production process, less quality control parameters, less operators, low risk of cross pollution, simple preparation process, less production processes, simple auxiliary materials and low cost. According to the estimation of authoritative experts, the comprehensive cost of hard capsules is 25-30% lower than that of tablets [5].
With the vigorous development of capsules, hollow capsules, as one of the main excipients, also have good performance. In 2007, the total sales volume of hollow capsules in the world has exceeded 310 billion, of which 94% are gelatin hollow capsules, while the other 6% are from non animal derived capsules, of which the annual growth rate of hydroxypropyl methylcellulose (HPMC) hollow capsules is more than 25%.
The substantial increase in the sales of non animal derived hollow capsules reflects the consumption trend of advocating natural products in the world. In the United States alone, for example, there are 70 million people who have “never eaten animal derived products”, and 20% of the total population are “vegetarians”. In addition to the natural concept, non animal derived hollow capsules also have their own unique technical characteristics. For example, HPMC hollow capsules have very low water content and good toughness, and are suitable for the contents with hygroscopicity and water sensitivity; Pullulan hollow capsule disintegrates rapidly and has very low oxygen permeability. It is suitable for strong reducing substances. Different characteristics make various hollow capsule products successful in specific markets and product categories.
REFERENCES
[1] La Wall, C. H., 4000 years of pharmacy, an outline history of pharmacy and the allied sciences, J. B. Lippincott Comp., Philadelphia/London/Montreal, 1940
[2] Feldhaus, F. M.: Zur Geschichte der Arzneikapsel. Dtsch. Apoth.-Ztg, 94 (16), 321 (1954)
[3] Französisches Patent Nr. 5648, Erteilt am 25. März 1834
[4] Planche und Gueneau de Mussy, Bulletin de I’Académie Royale de Médecine, 442-443 (1837)
[5] Graham Cole, Evaluating Development and Production Costs : Tablets Versus Capsugels. Capsugel Library
Post time: May-06-2022






