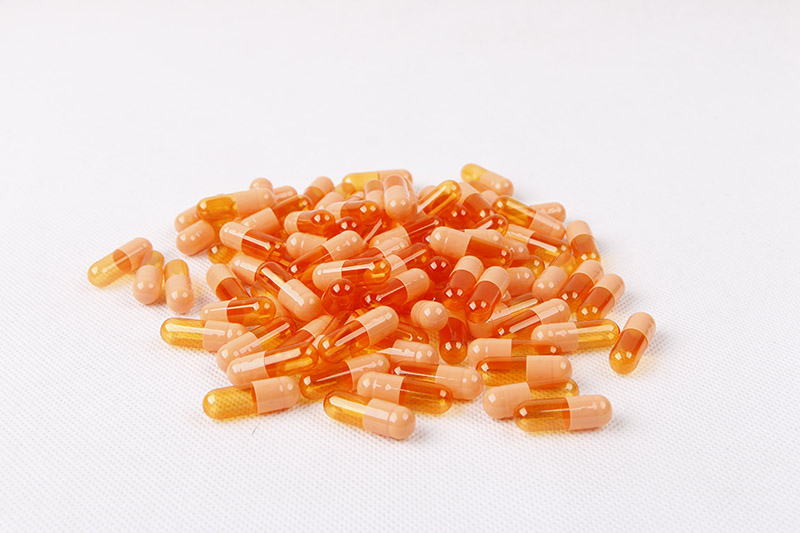
Hpmc Vegan Hard Empty Capsule
Description Details
What is HPMC capsule?
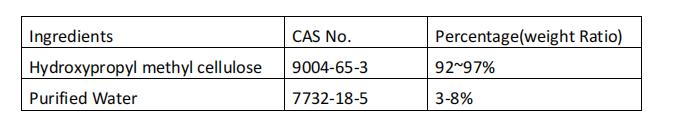
Hypromellose (HPMC) is a cellulose derivative that has been used in food and pharmaceuticals for more than 40 years. It is a widely used pharmaceutical polymer material with excellent performance. In pharmaceuticals, it has been widely used as thickener, film coating agent, pore-forming material for sustained-release preparations, hydrophilic gelling agent, and also as solid dispersant material to improve the stability of drugs and the bioavailability of poorly soluble drugs degree, etc.
The cellulose used to manufacture HPMC capsules is derived from trees, inert and free from animal source related issues. Low moisture content, ideal for moisture sensitive and liquid formulations.




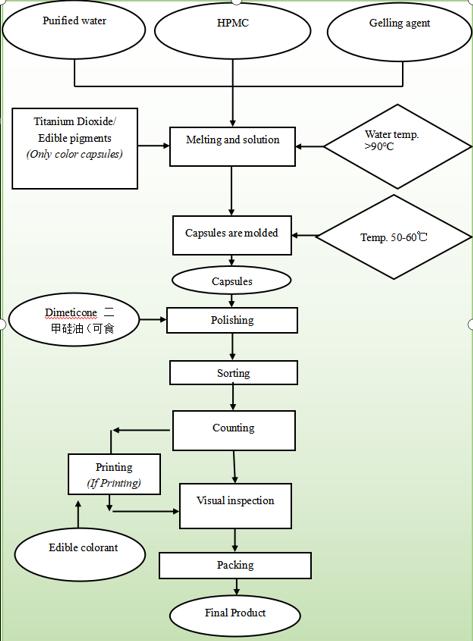
Production flow of Our HPMC Capsule
Our HPMC capsule is produced according to the strictest GMP standard. We provides safe, reliable and high-quality HPMC capsules for more than 3000 pharmaceuticals, health products and cosmetics at home and abroad.


Benefits of Our HPMC Capsule
It's our mission to protect your brand and reputation, our HPMC Capsule is derived from 100% plant raw materials
1.Natural & Health: Made From plant, Certified by Non-GMO, Halal Kosher and Vegsoc, GMP standard
2.Safety: No pesticide residues; No carcinogenic residue; No chemical additives; No virus risk; No cross-linking reaction
3.Appearance & Taste: Better thermal stability, Better taste, natural cassava sweetness Natural plant fragrance
4.Embrace Vegetarian Era: A compatibility with a broader range of fill excipients, enhancing bioavailability and stability
5.Quick Qelease After Ingestion:within 15 mins




Quality Control
Perform proactive and retrospective assess, control, communication and audit on the risks in the whole product life cycle in an attempt to better control and reduce the risk and safeguard the drug safety. The most advanced devices and instruments are equipped in quality control lab to make precise test and inspection.

Our Certificate






Specifications
|
Size |
00# 0# 1# 2# 3# 4# |
|||||
|
Color |
Customized |
|||||
|
Storage Condition |
Temperature:15℃~25℃ Humidity :35%~65% |
|||||
|
Package |
Customized |
|||||
|
MOQ |
5 million |
|||||
|
Type |
Description |
Length ±0.4(MM) |
Average weight |
Lock Length ±0.5 (MM) |
Outer Dia(MM) |
Volume(ML) |
|
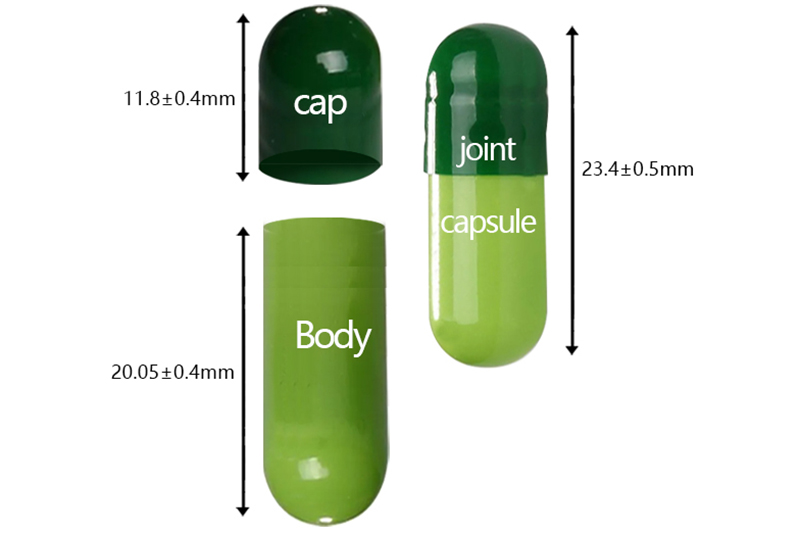
00# |
cap |
11.80 |
123±8.0 |
23.40 |
8.50-8.60 |
0.93 |
|
body |
20.05 |
8.15-8.25 |
||||
|
0# |
cap |
11.00 |
97±7.0 |
21.70 |
7.61-7.71 |
0.68 |
|
body |
18.50 |
7.30-7.40 |
||||
|
1# |
cap |
9.90 |
77±6.0 |
19.30 |
6.90-7.00 |
0.50 |
|
body |
16.50 |
6.61-6.69 |
||||
|
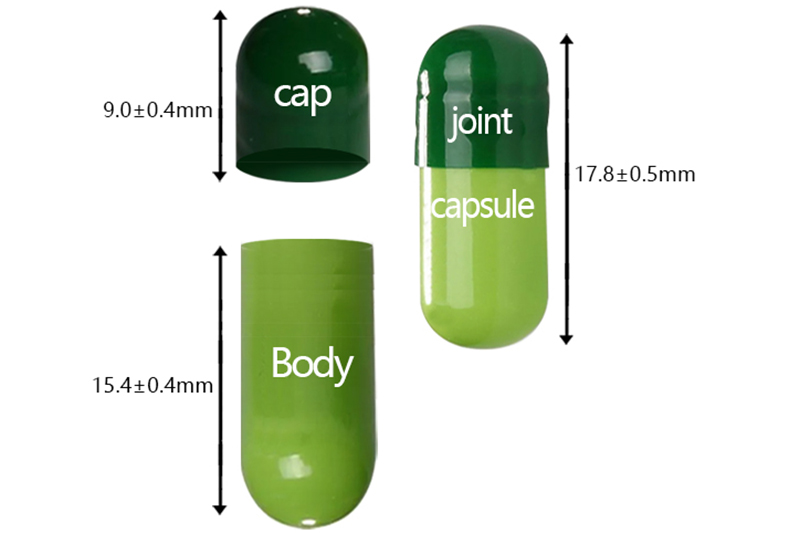
2# |
cap |
9.00 |
63±5.0 |
17.80 |
6.32-6.40 |
0.37 |
|
body |
15.40 |
6.05-6.13 |
||||
|
3# |
cap |
8.10 |
49±4.0 |
15.70 |
5.79-5.87 |
0.30 |
|
body |
13.60 |
5.53-5.61 |
||||
|
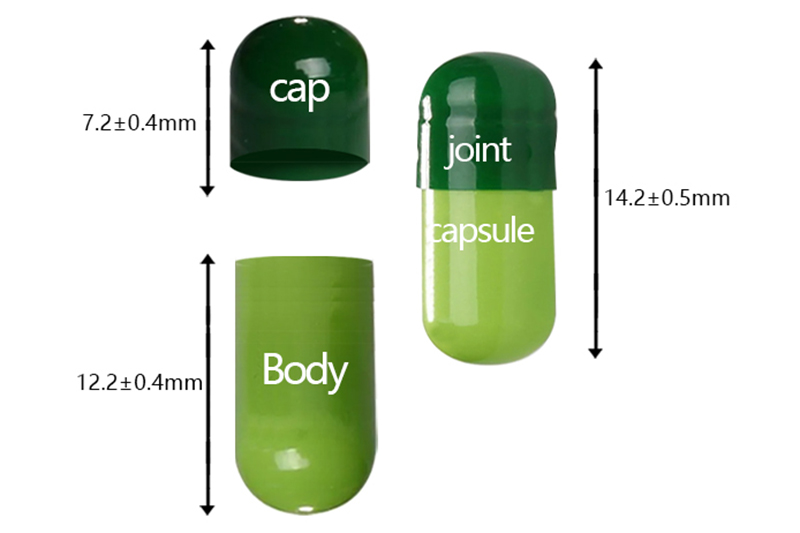
4# |
cap |
7.20 |
39±3.0 |
14.20 |
5.28-5.36 |
0.21
|
|
body |
12.20 |
5.00-5.08 |
||||
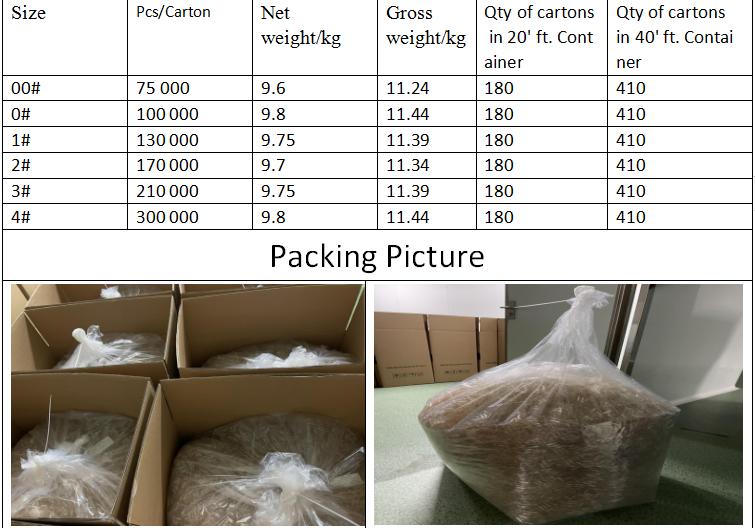
Reliable Empty Capsule Packing details
Storage precautions
1. Keep the Inventory temperature at 10 to 25 ℃; Relative humidity remains at 35-65%. 5 Years storage guarantee.
2. The capsules are supposed to be kept in clean, dry and ventilated warehouse, and are not allowed to be exposed to strong sunlight or humid environment. Besides, as they are too light to be fragile, the heavy cargo should not pile up
advantage of capsule
1. It is safer and riskless for animal infectious diseases. The cellulose used to manufacture HPMC capsules is derived from trees, inert and free from animal source related issues.
2. Low moisture content under 6%-7%, which is more applicable for moisture-sensitive and liquid formulations drugs.
3. HPMC Capsules are odorless and tasteless, easy to swallow and effectively mask taste and odor. The oral bioavailability of these capsules is identical to hard gelatin capsules.
4. Better stability enables the capsules to be stored 36 months without deterioration. It will not easily become crisp or deformed unless under extreme environment.
5. No risk for crosslinking reaction with aldehyde drugs. The thorough dissolving output brings the drug effect to the best.
6. It is almost acknowledged by all the groups with different cultural background and religion beliefs. There is no barrier to capsule promotion.
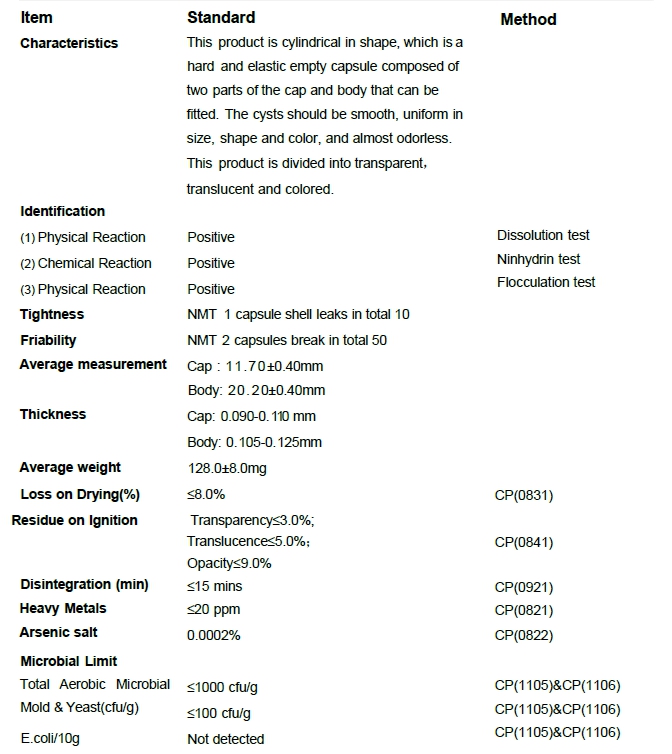
HPMC Capsule Specification
Specification sheet
HPMC Capsule
Hypromellose (HPMC) is a cellulose derivative that has been used in food and pharmaceuticals for more than 40 years. It is a widely used pharmaceutical polymer material with excellent performance. In pharmaceuticals, it has been widely used as thickener, film coating agent, pore-forming material for sustained-release preparations, hydrophilic gelling agent, and also as solid dispersant material to improve the stability of drugs and the bioavailability of poorly soluble drugs degree, etc.
The cellulose used to manufacture HPMC capsules is derived from trees, inert and free from animal source related issues. Low moisture content, ideal for moisture sensitive and liquid formulations.
HPMC Capsules are odorless and tasteless, easy to swallow and effectively mask taste and odor. The oral bio-availability of these capsules is identical to hard gelatin capsules.
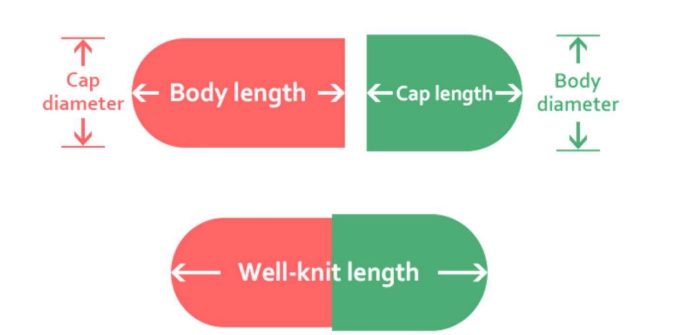
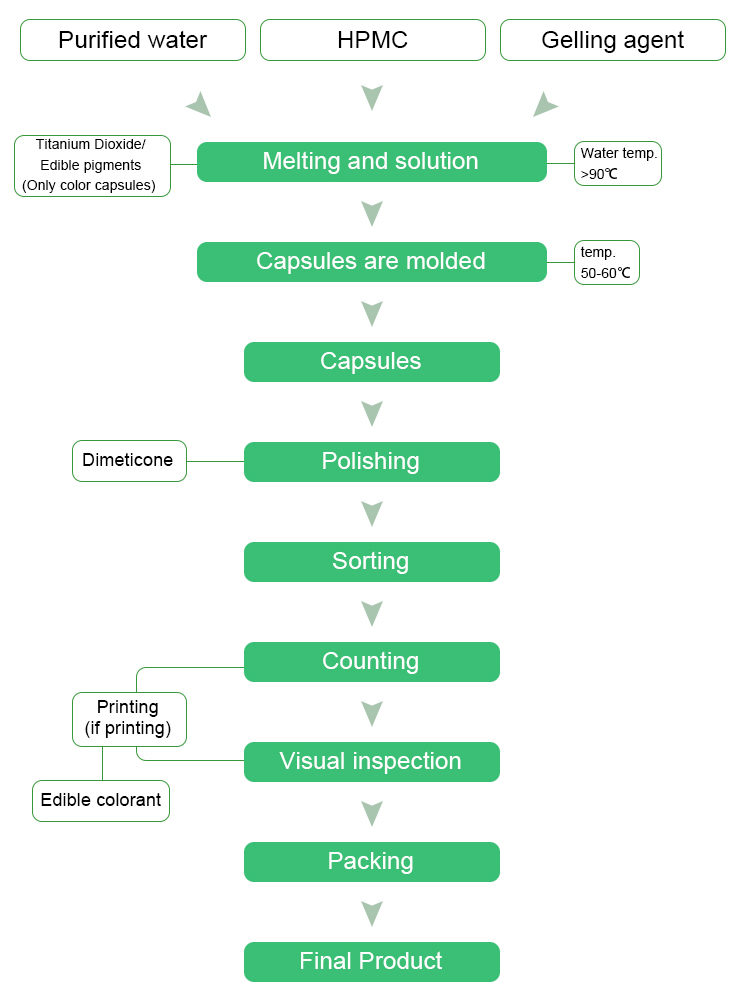
production process
Plant Capsule Production Process
quality system
1. We conduct strict control of raw material & products quality. The raw material of HPMC is based on natural wood fiber with GMO-free. The whole material quality system is guaranteed, being paid attention in details to guarantee quality evenness.
2. The whole manufacturing process is implemented with great dedication and full responsibility. The world-class automatic facilities are used skillfully by Competent Personnel, establishing an efficient and orderly GMP management system. Here displayed some core advanced equipment conforming to the highest pharmaceutical standard:
World-class Aseptic Room Facility
Cutting-edge Manufacturing Machines
Well-documented Monitoring System
Stringent Hygiene Standards
Climate and Humidity Diagnostic Equipment
3. The quality assurance is absolutely trustable. Regular and planned hands-on workshops addressing the training needs enable us to maintain consistency. So no defective capsules are produced under such thorough inspection and continuous monitoring, as each step is cautiously reviewed in every management to continue suitability.
Safe Storage & Packing Condition
Storage precautions:
1. Keep the Inventory temperature at 10 to 25 ℃; Relative humidity remains at 35-65%. 5 Year’s storage guarantee.
2. The capsules are supposed to be kept in a clean, dry and ventilated warehouse, and are not allowed to be exposed to strong sunlight or humid environment. Besides, as they are too light to be fragile, the heavy cargos should not pile up.
Packaging requirements:
1. Medical low-density polyethylene bags are used for inner packaging.
2. To prevent damage and moisture, the outer packing uses 5-ply Kraft paper dual corrugated structure packing box.
3. Two outer packing specifications: 550 x 440 x 740 mm or 390 x 590 x 720mm.